
1999 Molecular Chirality Award Winner; Kazuhiko Saigo Ph.D.
(Interviewer; Erika Odaka)

INTRODUCTION
I would like to explain briefly our studies on the optical resolution of racemates though crystallization. As you know, there are mainly two methods for obtaining enantiopure compounds, the optical resolution of racemates and the asymmetric transformation of prochiral or achiral compounds. Although there are many reports for the studies on the asymmetric transformation, we are widely applying the optical resolution of racemates even at present for obtaining enantiopure compounds in industrial scales as well as laboratorial scales. For the optical resolution of racemates, there are several methods using physical, chemical, or biological technique. Among them, the preferential crystallization and the diastereomeric salt formation are commonly performed through crystallization for the optical resolution of racemates. Although both methods have a long history for about 150 years, no one knows the mechanisms for the chiral discrimination of the enantiomers of racemates during the crystallizations. Thus, we are interested in the elucidation of the mechanisms and in development of some criteria on the basis of the mechanisms in order to carry out the two methods more efficiently.
THE PREFERENTIAL CRYSTALLIZATION
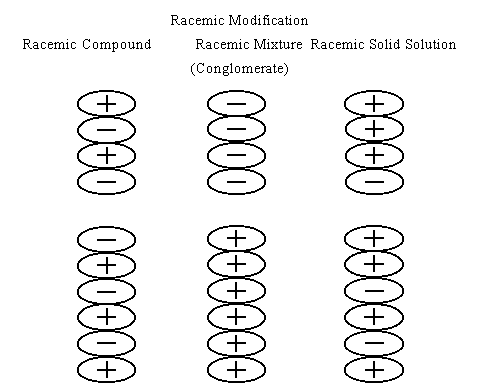
There are three crystal forms for racemates. They are racemic compound, conglomerate, and racemic solid solution. Usually, racemates crystallize in the form of racemic compound. However, some racemates, less than 10% of all of racemates, crystallize in the form of conglomerate. As shown in this figure, a conglomerate is a mixture of single crystals of both enantiomers. So if you add a small amount of the crystals of one enantiomer of a conglomerate, for example the minus crystals, as seeds to a supersaturated solution of the conglomerate, the minus crystals deposits in a large quantity. In the next stage, upon adding a small amount of the plus crystals to a supersaturated solution prepared from the filtrate and the conglomerate, you can obtain the plus crystals in a large quantity. Thus, you obtain both enantiomers from a racemate by alternative seeding.
The case that a racemate is a conglomerate is very few, less than 10%. This
means that we can carry out the preferential crystallization of a racemate
by chance, although the preferential crystallization if fascinating for
obtaining both enantiomers from a racemate by a simple operation. So we
considered that we have to develop some criterion for derivatizing a
racemate into a conglomerate with high probability. After the accumulation
of several considerations for the results on the derivatizations, we had an
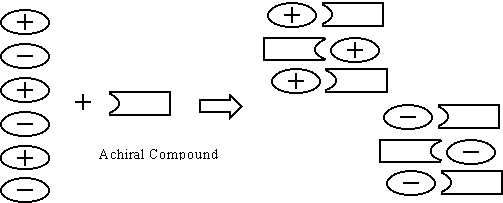
idea that if we add a flat achiral reagent to a racemate, which strongly
interacts with the racemate, the achiral reagent will works as a partition.
The partition will make the interaction between the plus and minus
enantiomers of the racemate small enough, and the same enantiomeric
molecules may aggregate to form a conglomerate. Based on this idea we
initially carried out the optical resolution of 1-phenylethylamine by the
preferential crystallization upon derivatizing 1-phenylethylamine to the
salts with achiral flat acids. Here, I would like to emphasize that these
salts are not diastereomeric but enantiomeric.
There are mainly three methods in order to know whether a racemate is a conglomerate or not. They are the comparisons of the melting point, solubility, and IR and/or XRD spectrum between the racemic and optical active forms of the racemate. Usually, in the case of a conglomerate, its optically active form has lower solubility and higher melting point than the corresponding racemic form, and shows the same IR and/or XRD spectral pattern as that of the racemic form. Anyway, the cinnamic acid salt satisfied the three conditions. So we considered that the salt of 1-phenylethylamine with cinnamic acid should be a conglomerate. Then we tried to separate the salt into both enantiomers. After we prepared a supersaturated solution of the salt, coexisting 1-phenylethylamine HCl salt as a buffer, we added a small amount of the minus seeds to the solution to obtain 3.4g of the minus crystals with 80% optical purity. Then we added the racemate to the filtrate to prepare a supersaturated solution and seeded a small amount of the plus salt to obtain the plus crystals with 87% optical purity in a large quantity, and so on. Thus, we could obtain alternatively both plus and minus salt crystals in large quantities. Finally we recrystallized the both plus and minus salt crystals and obtained the enantiopure salts of 1-phenylethylamine with cinnamic acid. Treatment of the salts with an alkali solution gave us enantiopure 1-phenylethylamine.
We next considered that the comparison of the molecular shape of 1-phenylethylamine and cinnamic acid, which form a conglomerate, would give us valuable information for derivatizing a racemate into a conglomerate. In order to know the relationship in molecular shape between the racemic amine and the achiral acid, we prepared the CPK models of the molecules and compared with each other. As a result, we found that the molecular length of cinnamic acid is very similar to that of 1-phenylethylamine. In contrast, in the case of benzoic acid, which could not convert 1-phenylethylamine into a conglomerate, its molecular length is shorter than that of 1-phenylethylamine.
So we considered that one of important factors for derivatizing a racemate to a conglomerate is the relative molecular length between the racemate and the derivatizing agent. Then we tried to apply this criterion for derivatizing another useful racemate to a conglomerate. At first we selected this newly designed compound, erythro-2-amino-1,2-diphenylethanol as a target. We designed this compound on the basis of the molecular structure of norephedrin. Norephedrin is a natural product, which is widely used in optical resolution as a resolving agent and in asymmetric synthesis as a chiral auxiliary. However, we hardly can use this compound in a large industrial scale, because this is a pro-drug having limitation in usage. So we tried to develop a new compound, which can behave as norephedrin. We considered to introduce a phenyl group in the place of methyl group to the norephedrin skeleton. Because as you know a phenyl group is larger than a methyl group and would make chiral environment effective for asymmetric induction when we use it as a chiral auxiliary, and the phenyl group may contribute to the increase of the cristallizability of the corresponding diastereomeric salts when we use it as a resolving agent.
Of course erythro-2-amino-1,2-diphenylethanol is not a conglomerate but a racemic compound. Then, we tried to convert it into a conglomerate upon derivatizing it to a salt with a achiral acid. As a result we found that the cinnamic acid salt of erythro-2-amino-1,2-diphenylethanol again satisfies the three conditions. Then we tried to resolve the racemic salt into the single enantiomers. Please look at this figure. If you seed alternatively plus, minus, plus, minus crystals in small quantities, you can obtain plus, minus, plus, minus salts in large quantities with high optical purity.

On the basis of the criterion we proposed, we could find that these six racemic salts are conglomerates within two years. Namely, when we converted these racemates into the salts with these achiral compounds, respectively, the salts satisfied the three conditions, we could resolve the salts into both enantiomers by seeding, respectively. At that time, there were about 240 reports on conglomerates, Professors Jacques, Collet, and Wilen, France, noticed in their book that there known 240 conglomerates in the world. The optical resolution of conglomerates has a 150-year history, but only 240 conglomerates were reported. In contrast, in our case, within two years we found six conglomerates. This fact indicates that our criterion is appropriate. Namely the relative molecular length between a racemic acid or amine and a basic or acidic derivatizing agent is an important factor for derivatizing the resulting salt into a conglomerate. Moreover, I didn't explained in detail, the similarity in shape between the molecules is also important.
As I explained, we proposed criteria for derivatizing a racemate into a conglomerate. But no one knows how to choose a suitable achiral derivatizing agent. I mentioned that the relative molecular length and the similarity in shape are important. So we next considered that the prediction of a conglomerate crystal on the basis of computer chemistry would be valuable.
For the prediction, we initially carried out the X-ray crystallographic analyses of conglomerates and racemic compounds in order to extract the similarity in the crystal structures of conglomerates and to compare the structures with those of racemic compounds. Thus, we prepared a plenty kinds of salts of racemic amines and achiral acids. As a result, we found that in order to obtain a conglomerate crystal it is important to form a 21-column in the crystal.
Namely, we found that a conglomerate essentially should have a 21-column. However, there is another pattern of hydrogen bonds in some of the salts prepared. It is an i-column. The i-column consists of both enantiomers of the racemate as well as the derivatizing agent. This means that the salt having an i-column in the crystal is no longer conglomerate. Anyway, we also found from the X-ray crystallographic analyses that the relative molecular length is very important. Let's say, a 21 column is like a spring. For springs, there are right-handed and left-handed springs. If you mix the right-handed and left-handed springs, they have a possibility to form locking and/or independent aggregations, depending on the pitch and thickness of the springs. However, if the relative molecular length and shape between a racemate and a derivatizing agent are similar to each other, the springs become like rods without any space for making locking due to the close packing of the molecules of the racemate and derivatizing agent along a 21-column, and subsequently the springs would form independent aggregations with some higher possibility. In contrast, if the relative molecular length and shape are largely different, the surface of the rods becomes rough and/or the helix becomes loose to give some space for locking.
DIASTEREOMERIC SALT FORMATION Another important method for obtaining enantiopure compounds from racemates is the diastereomeric salt formation. These enantiopure drugs and pro-drugs are examples recently obtained by the diastereomeric salt formation. Such valuable drugs and pro-drugs are resolved into each enantiomer by the diastereomeric salt formation even at present.
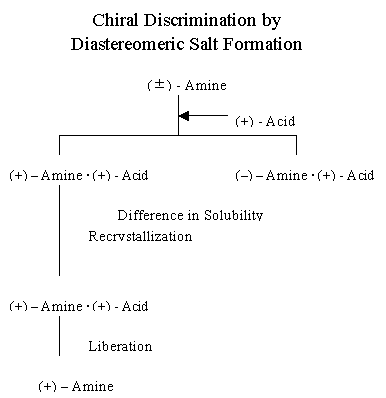 This figure shows the flow-chart of the diastereomeric salt formation. When
you want to resolve a racemic amine, you add an optical pure acidic
resolving agent and you obtain a pair of the diastereomeric salts. Here, if
(+)(+) salt is less soluble than (-)(+) salt, you can obtain (+)(+) salt in
a pure form upon repeating recrystallization. Then, upon decomposing the
pure salt, for example by treating with an alkali solution, to liberate the
amine, you obtain the enantiopure (+)-amine. The fundamental principle of
the optical resolution by the diastereomeric salt formation is very simple.
The resolution depends on the deference in solubility between a pair of
diastereomeric salts.
This figure shows the flow-chart of the diastereomeric salt formation. When
you want to resolve a racemic amine, you add an optical pure acidic
resolving agent and you obtain a pair of the diastereomeric salts. Here, if
(+)(+) salt is less soluble than (-)(+) salt, you can obtain (+)(+) salt in
a pure form upon repeating recrystallization. Then, upon decomposing the
pure salt, for example by treating with an alkali solution, to liberate the
amine, you obtain the enantiopure (+)-amine. The fundamental principle of
the optical resolution by the diastereomeric salt formation is very simple.
The resolution depends on the deference in solubility between a pair of
diastereomeric salts.
As I told you, you add a resolving agent for the optical resolution by the diastereomeric salt formation. But no one can give an answer to a question how to choose a resolving agent suitable for a given racemate.
Usually we choose a suitable resolving agent by trial-and-error, because there is no criterion for selecting a suitable resolving agent at present. Actually, the study on the optical resolution by the diastereomeric salt formation is strongly target oriented; when one researcher want to resolve this racemic amine, the researcher prepare many kinds of salts with enantiopure acids and choose the best one.
Such situation prompted us to develop some criterion for choosing a suitable resolving agent. We then considered that we had to carry out a systematic study on the optical resolution by the diastereomeric salt formation in order to overcome target-oriented situation. Moreover, on the basis of our experience concerned with the preferential crystallization as I explained, we also considered that the elucidation of the structural correlation between racemates and resolving agents would be valuable for the development of the criterion.
In order to compare different results to each other, we set the conditions for crystallization as similar as possible to each other. At first we tried the optical resolution of 1-arylethylamines with enantiopure mandelic acid, because mandelic acid is a widely applicable resolving agent and because 1-arylethylamines are simple series of chiral amines and some of them are variable as pro-drugs.
This table shows the results. Here, we introduce value of resolution efficiency defined as shown here. Both enantiomeric excess and yield are very important, because high yield with low purity and low yield even with high purity are meaningless in the optical resolution by the diastereomeric salt formation; high yield with high purity is ideal. So, thus defined resolution efficiency is very valuable for the comparison of results on the optical resolutions of different racemates by the diastereomeric salt formation. These three racemates, having no substituent or an o-substituent, are resolved with high resolution efficiency, while the p-substituted amines could not be resolved. Thus, it seems that there is some correlation between the position of the substituent and the resolution efficiency. Then, we compared the molecular lengths. We every time compare the molecular lengths!!
These are mandelic acid molecules, and these are the amine molecules. In the case of amines having no substituent, the molecular lengths are similar to each other. For amines having an o-substituent, there are two conformers but both lengths are similar to that of mandelic acid. In the case of amines having a p-substituent, the length is longer than that of mandelic acid. When the molecular lengths of the amine and mandelic acid are similar, the resolution resulted in high efficiency, while the molecular lengths are different, the resolution resulted in low efficiency. There are two conformers for m-substituted amines; the molecular lengths are similar and longer. So the resolution efficiencies are case by case!
We next tried the X-ray crystallographic analyses of the salts, because we considered that the resolution efficiencies should depend on the difference in solubility between the pair of diastereomeric salts and that the difference in solubility should arise from the difference in crystal structure between them.
Then we carried out the X-ray crystallographic analyses of the less-soluble salts deposited and more-soluble salts remained in solutions. I would like to explain only the patterns of the salts formed.
This figures show the schematic representations of the crystals of the salts. In the case of high-resolution efficiency was achieved, the less-soluble salts, deposited salts, have commonly two characteristic factors for the stabilization of the crystals. There exists a supramolecular sheet consisting of 21-columns, as can be seen here and here. These are 21-columns, existing from front to back. Finally, the columns form the sheet by other hydrogen bonds between the columns. Moreover, the surfaces of the supramolecular sheet are planar. The formation of a supramolecular sheet should be favorable from the viewpoint of hydrogen bonding interaction, and the planar surfaces would be favorable for the close packing of the sheets by Van der Waals interaction. Thus, we can say this salt is stabilized by two factors.
In contrast, the more-soluble salts are stabilized by only one of the two factors, although there are two stabilizing modes. Namely there is a supramolecular sheet but its surfaces are irregular, or there is no 21-column although some sheet of hydrogen bonding with planner surfaces exists. Thus, these salts stabilized by only one factor. The salt stabilized by two factors should be more stable and less soluble than the salt stabilized by one factor.
We also carried out the X-ray crystallographic analyses for the pair of diastereomeric salt, which resulted in low-resolution efficiency. This figure shows the crystal structure of the deposit salt. There is a sheet with planner surfaces, so as to stabilize by two factors. But, the columns consist of both enantiomers of the amine. These are the amine molecules. These are the plus amine molecules in this column, but the neighboring column consists of the minus amine molecules. The plus, minus, plus, and minus amines; so this crystal is a racemic compound. We call this type of compound as a double salt.
We prepare separately two diastereomeric salts. One of them satisfies the formation of a supramolecular hydrogen network, and the other satisfies the formation of planner surfaces. So in the solution, the two diastereomeric salts are stabilized by only one of the two factor, and the actual deposited crystal includes both plus and minus amine molecules in order to stabilize the crystal by the two factors. Thus, there are two factors for the stabilization of the diastereomeric salts, The solubility as well as the stability of the salts is determined by the two factors.
On the basis of these results, we proposed a criterion for choosing a suitable resolving agent for a given racemate that the relative molecular length is very important; we have to choose a resolving agent having a molecular length similar to or a little longer than that of a racemate.
Then we applied this criterion to the development of new resolving agents. As shown in this figure, one of the diastereomeric acids satisfied the formation of a supramolecular sheet but the surfaces are not planar. So we considered that if we introduce a subsequent at the p-position of the phenyl group of mandelic acid, this subsequent should occupy the vacancy at the surface. On the basis of this idea, we designed p-methyl- and p-methoxylmandelic acid as a new resolving agents.
This table shows the results of the optical resolution using mandelic acid and its derivatives. This is not so good. But, anyway the resolution efficiencies are highly improved by introducing a substituent at the p-position of the phenyl group of mandelic acid to make the surfaces planar.
This is the crystal structure of one of the less-soluble diastereomeric salts. In this case, the substituent occupied the vacancy at the surfaces to make the surfaces planar, as we expected.
On the basis of similar crystal engineering, we designed 2-naphthylglycolic acid. This compound has a naphthyl group in the place of the phenyl group of mandelic acid. We expected that this part of the naphthyl group play a role as a substituent.
In order to demonstrate the resolution ability of 2-naphthylglycolic acid, we carried out the optical resolution of p-substituted 1-arylalkylamines, which could not be resolved by mandelic acid. As you can see from this table, the resolution efficiencies are very high. Moreover, we could resolve these compounds; as you can imagine, these substituents are very large but they were commonly resolved into the enantiomers by using 2-naphthylglycolic acid.

Then we carried out X-ray crystallographic analyses. As a result, we found that there is another factor for the stabilization of the crystals. As you can imagine, the p-cyclohexylated amine is very large, so the surfaces should be no longer planar. But we succeeded the optical resolution of it by the diastereomeric salt formation. This means that there is another factor. In conclusion, we found that it was CH-pi interaction. An aromatic C-H and an aromatic pi can interact. In the less-soluble salts, CH-pi interaction works very strongly to realize herringbone packing, while in the more-soluble salts, there exists very weak or no CH-pi interaction.
In other cases, there observed different hydrogen bonding patterns in a supramolecular hydrogen-bonded sheet consisting of 21-columns for less- and more-soluble salts. This is an amine molecule; there are four hydrogen bonds in the less-soluble salt. As you know, the ammonium group of a primary amine is a three-hydrogen donor. Why are there four hydrogen bonds in this case? It means that there is a so-called 3-point hydrogen bond, although a hydrogen is usually two-point as is seen in this more-soluble salt. It is well known that a 3-point hydrogen bond is more stable than a 2-point hydrogen bond. This difference would make the difference in stability, namely in solubility, between the pair of the diastereomeric salts large enough.
In conclusion, there are three factors for stabilizing diastereomeric crystals. The first factor is hydrogen bonding interaction to form a supramolecular sheet consisting of 21-columns. In some cases, the formation of a 3-point hydrogen bond in a 21-column is important. The second factor is C-H pi interaction between the aromatic groups of a racemate and a resolving agent. The third factor is Van der Waals interaction between the supramolecular sheets. They contribute to the stabilization of the crystal in this order; the first, the second, and the third!!
The next explanation may be complicated. I explained our studies on the optical resolution of racemic amines with acidic resolving agents. The successful results prompted us to carry out a similar study under opposite situation. Namely, we tried the optical resolution of racemic acids with amino resolving agents. In the cases of alpha-hydroxy acids we used as acidic resolving agents, the carboxyl group forms a salt with an amino group, and the hydroxy group contributes to the formation of a sheet. So we considered amino resolving agents we targeted, should have a hydroxy group other than an amino group with an expectation that the amino group forms salt with a carboxyl group in racemates and the hydroxy group may contribute to the formation of a sheet. On the basis of this consideration and expectation, we carried out the optical resolution of racemic acids with enantiopure amino alcohols.
As a result, we could not observe the formation of a supramolecular sheet at present. However, we got similar results that the difference in stability, namely in solubility, is determined by three factors, hydrogen bonding interaction, CH-pi interaction, and van der Waals interaction in this order.
As exemplified, systematic studies on the optical resolutions by the preferential crystallization and by the diastereomeric salt formation are very valuable for the elucidation of resolution phenomena and for the design of suitable derivatizing and resolving agents. I hope that our studies can contribute to the development of some criteria for derivatizing a racemate to a conglomerate and for selecting a resolving agent suitable for a given racemate.