1999 Molecular Chirality Award Winner; Masakatsu Shibasaki Ph.D.
(Interviewer; Erika Odaka)
(Please explain your study briefly)
At a moment, one of the most important areas in organic chemistry is how to synthesize organic molecules in an enantioselective manner. Of course traditionally, optically active compounds can be obtained by classical resolution method or by using stoichiometric amounts of chiral compounds. This means that to prepare one mol optically active products we have to utilize one mol optically active compounds. This is not so efficient, therefore last decade or two decades the most important way has been recognized to develop very efficient, very practical catalytic asymmetric syntheses.
History of Asymmetric Catalysis
Catalytic asymmetric synthesis means that, to synthesize, for example
one mol of optically active compounds, the use of, for example 0.1 mol
or 0.01 mol asymmetric catalyst is enough like enzyme. I think that this
field would deserve Nobel Prize in near future, and so far, in the epoxidation
or hydrogenation reaction of molecules, very practical and very efficient
catalytic asymmetric reactions have been developed by Prof. Sharpless in
Scripps Institute in the United States and Prof. Noyori in Nagoya University.
However, in the case of asymmetric carbon-carbon bond-forming reaction
still very practical asymmetric catalysis has not been developed. And of
course many chemists in the world are involved in this field, and all the
chemists utilize the asymmetric catalysts which can activate one of the
substrates. Reaction can take place between A substrate and B substrate
to form C product.
Bifunctional Asymmetric Catalysis
In contrast to that strategy, our chemistry is very different. We are looking at enzyme very much. Enzyme is, as you know big molecule, and all the reactions are very very nicely organized. If we consider the mechanism of reaction promoted by enzyme, it’s clear that enzyme controls both substrates, A and B, in an asymmetric pocket, to produce optically active pure product very very efficiently. However I would say there are still drawbacks in enzymes. One of the shortcomings is enzymes have extremely high substrate specificity. Can you understand substrate specificity? Substrate high specificity means that only A and B can be utilized for the starting material. So, the first starting point in our research is to think about "Is it possible to design and prepare small molecule asymmetric catalysts with multifunction?, multifunction means to activate both A and B, like enzyme, and hopefully broader substrate generality, broader substrate generality means that many starting compounds can be utilized as a substrate.” Our research features multifunctional asymmetric catalysis. Although there are so many excellent chemists in the world, only our group is emphasizing multifunctional or bifunctional asymmetric catalysis that means to activate and control both substrates. Based on such a point of view, I want to say, our chemistry or science is recognized from the world, due to very high originality. About 10 years ago, we succeeded in developing such a kind of asymmetric catalyst as a first time, which was appreciated by reagent of the year by Fluka, Switzerland, in 1996.
Lanthanide-Alkali Metal-BINOL Complex
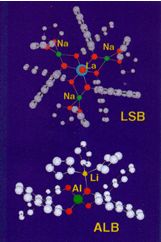
This type of asymmetric catalyst contains lanthanide, alkali metal and bi-naphthol. This is an X-ray structure of lanthanum-sodium bi-naphthol complex. There are so many lanthanides, so we can change very easily lanthanide, and also there are so many alkali metals such as lithium and potassium. So by suitably choosing both metals, we can prepare a variety of this type of asymmetric catalyst. In every asymmetric catalyst, as you expect, bond length is slightly changed, leading to slight changes of asymmetric space. This catalyst exhibits Bronsted basicity. Bronsted basicity can function to abstract H+ as a base to activate substrates. And also this catalyst exhibits Lewis acidity. Lewis acidity means that, for example ketone or aldehyde coordinate to Lewis acid through lone pair coordination. That means it controls the position of another substrate and to activate it. Lanthanide has f orbital. That's why it can receive at most 12 ligands, very big element, you know. So, this catalyst can control a variety of catalytic asymmetric reaction. For example, we developed catalytic asymmetric nitroaldol reaction. Nitroaldol reaction means, reaction of aldehyde with nitromethane to form nitroaldol. You can see here, this is nitroaldol. Of course so far many people have performed this reaction to get racemic nitroaldol, and no catalytic asymmetric reaction has been developed. We have succeeded in developing the first example of catalytic asymmetric nitroaldol reaction using our new multifunctional asymmetric catalyst. And in several cases, you can obtain products in almost optically pure form. As shown here nitromethane can be activated by the reaction with this lithium naphthoxide. This lithium naphthoxide can function as a Bronsted base, to abstract H+ from nitromethane, and at same time, aldehyde can coordinate lanthanide, forming self assembled complex. And from this intermediate carbon-carbon bond-forming reaction can take place here, giving highly optically active products. This is just something like enzyme model, because this is a very small molecule, low weight molecules.
(To make this molecule, you use the lithium one, not the sodium one.)
Yes that is a very good question. Let me continue to explain. In this case, you can synthesize this nitroaldol and in a conventional manner this can be converted to this therapeutically useful beta-blocker. Beta-blocker is one of the therapeutically useful drugs. In this case, the presence of lithium is essential. There is another reaction, such as Michael reaction. Michael reaction means the addition of malonate derivative to cyclohexenone, and in this case chirality is produced here. To promote this reaction very efficiently, we found the use of sodium, instead of lithium, was essential. In this case, again, transition state is very similar. Sodium naphtoxide moiety can abstract H+ from here, and this activated form is located in an asymmetric space of this molecule. And cyclohexenone carbonyl coordinates to lanthanide. Thus the position is controlled.
(So this catalysis catches both of the molecules, then combine them, and the specific molecule.)
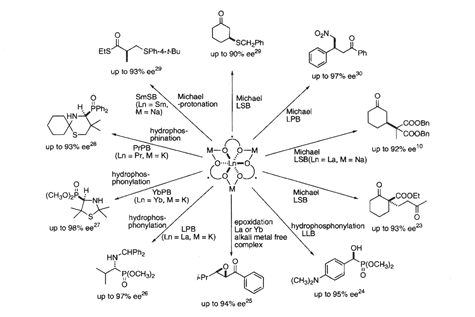
For some particular reaction, the selection of the best lanthanide and the best alkali metal is very very important. So as mentioned already, in the case of nitroaldole reaction, the use of lanthanum-lithium bi-naphthol complex is very essential. Also we developed catalytic asymmetric aldol reaction. Aldol reaction is very simple reaction, reaction of aldehyde and ketone. So in this case, acidic proton should be deprotonated here. This is a typical structure of aldol. In this case, also the use of lanthanum-lithium bi-naphthol complex is very very important.
However, as mentioned already, in the case of Michael addition, we have to change alkali metal from lithium to sodium. And the best catalyst is lanthanum-sodium bi-naphthol complex.
(So, LSB?)
As shown here, we have developed a variety of asymmetric catalytic reaction. For example, this is a reaction to prepare optically pure amino phosphonic acid. That is an analogue of amino acid. So this is a very very important molecule for the synthesis of peptide mimetic. To synthesize this compound we introduce phosphite functionality to imine, and of course here, chirality is produced. In this case quite interestingly, the use of lanthanide-potassium bi-naphthol is the most efficient. As demonstrated here, to develop efficient reactions, we have to select the best combination.
So far when I presented such a kind of chemistry at many places in the world, many people asked, is it possible to expect rationally, which combination is the best? Actually it's so far very difficult. But based on many many experimental results, we know the tendency. In the case of reaction of aldehyde, the use of lanthanide-lithium bi-naphthol combination is the most efficient. And in the case of Michael reaction, 1-4 type addition reaction, combination of lanthanide- sodium bi-naphthol complex, is the most efficient. Moreover, in the case of reaction using imine, as shown here, use of lanthanide-potassium bi-naphthol complex, which we call LPB, is the most efficient.
By choosing metals properly you can develop a variety of very efficient catalytic asymmetric reaction. One of the characteristic examples is shown here. This is the compound, which is very important component of HIV protease inhibitor, for the therapeutically useful molecule for AIDS. "Kanegafuchi" chemical company in Japan has already utilized our catalyst for the synthesis of this molecule. This molecule is erythro-AHPA. Also several companies are now developing industrial scale synthesis of in particular pharmaceutical intermediate, using our asymmetric catalyst.
That
is one of the multifunctional asymmetric catalysts showing Bronsted basicity,
as well as Lewis acidity. Moreover, in that field you can develop a variety
of other types of catalyst. For example, as a center metal instead of lanthanide,
you can utilize group 13 elements, such as aluminum and gallium. And of
course in this case you can prepare aluminum-lithium bi-naphthol complex,
which we call ALB. Also you can replace this aluminum with gallium, and
GaLB is formed. Of course also in this case you can prepare a variety of
combination. For example, aluminum-lithium, aluminum-sodium, aluminum-potassium,
and also gallium-lithium, gallium-sodium, gallium-potassium.
(What kind of molecule does this type of catalysis works?)
This type of catalysis is very very useful for, for example, catalytic asymmetric epoxide opening reaction. As shown here, (http://www.f.u-tokyo.ac.jp/~kanai/rxn/epoxide.html) this is a prochiral molecule. If you can open up this epoxide with for example phenol derivative or thiol derivative, resulting product is optically active. And this can be synthesized very efficiently, and already Prof. Evans at Harvard University is utilizing this product for their asymmetric catalysis.
(Why lanthanide or why aluminum is important for this Lewis acidity?)
Of course both metals can function as a Lewis acid, that can receive electron. Since there is the structual difference between lanthanide containing heterobimetallic catalyst and group 13 elements containing heterobimetallic catalyst, asymmetric space should be different. Some asymmetric space would be more suitable for the some reaction. That's why still we have to try many many experiments to find the some special efficient catalytic asymmetric reaction.
(So, What you are saying is that these metals can become the Lewis acids but not yet known why so suitable for special reaction.)
To understand precisely, probably collaboration with computational chemists or molecular calculation chemists are definitely required. We are going to collaborate with such a kind of professor in terms of mechanistic clarification.
Lewis Acid-Lewis Base Asymmetric Catalysis
As shown here, (http://www.f.u-tokyo.ac.jp/~kanai/rxn/CNSi.html) still we are continuing this type of chemistry to develop more useful reactions. About a couple of years ago, we succeeded in developing very very different bifunctional asymmetric catalyst. This shows Lewis acidity as well as Lewis basicity. Lewis basicity means just donation of electron to electron deficient one. The previous catalyst shows Bronsted basicity as well as Lewis acidity. Lewis acidity again controls the position of carbonyl or imine functionality also in new catalysts. However, in the new catalyst Lewis base can function by the donation of electron to electron deficient orbital, again to control the position of another substrate and activate it. So we have developed two types of bifunctional asymmetric catalysis. One is the catalyst showing Bronsted basisity as well as Lewis acidity. And another one exhibits Lewis acidity as well as Lewis basicity.
As the first target reaction, we chose catalytic asymmetric cyanosilylation of aldehydes. Our idea is as shown here aldehyde can be activated by the coordination to aluminum, and position is controlled. This is just a catalyst every chemist in the world is trying. In contrast we have introduced Lewis basic moiety that is phosphine oxide. Phosphine oxide can coordinate to silicon. Silicon has electron deficient d orbital. That's why phosphine oxide electron can coordinate to silicon. That means the position of trimethylsilyl cyanide is controlled and further accelerated. And as shown here, in every case almost 100%! enantiomeric excess can be obtained. Definitely this is the most general and most efficient cyanosilylation of aldehydes.
This is a chemistry of last year, and by extending this type of concept,
we have also developed catalytic asymmetric Strecker type reaction. Strecker
type reaction leads to a synthesis of amino acid. And also quite recently
we have developed catalytic asymmetric Reissert type reaction. Reissert
type reaction means introduction of cyano functionality, quinoline or isoquinoline.
The reaction is very very useful for the synthesis of pharmaceuticals.
So recently, we are now turning our interest to this type of asymmetric
catalysis. We are paying much more attention to this type of catalysis.
Optically active space is formed from bi-naphthol, and this phosphine oxide moiety is connected to binaphthol, and can function to control the position of trimethylsilyl cyanide and activate it.
(Not the lithium, but the phosphine oxide?)
If we explain very simply, new catalysis contains phosphine oxide, instead of lithium or sodium or potassium. That's why, leads to the development of very very different type of catalytic asymmetric reactions. This is a very rough explanation of our bi-functional asymmetric catalyst.
I want to emphasize, one more point. Many chemists involved in this field, just develop this type of catalyst and to develop only catalytic asymmetric reactions. But, I am very much interested in the application of this asymmetric reaction to the synthesis of the biologically significant compound.
This is a quite exciting compound named epothilone, as an anticancer drug. And many pharmaceutical companies and many medicinal chemists are very much interested in the development of this new molecule, as a new anticancer drug. We have to prepare this molecule in large amount to supply as a drug.
To synthesis this type of compound, of course we have to control the chirality. We succeeded in the synthesis of the molecule in the optically pure form. We utilized two type of reaction, developed in our group. This moiety can be constructed using this type of Lewis base-Lewis acid bi-functional asymmetric catalysis. And this area can be constructed using multifunctional catalyst showing Bronsted basicity as well as Lewis acidity, namely catalytic asymmetric aldol reaction.
All the time we are making so much effort to apply our asymmetric catalysis to a synthesis of biologically significant compound. Probably due to the fact I am a professor of pharmaceutical science. There are so many research groups in this field. But, I would say probably our group is one of the most characteristic groups because we are working in new area of asymmetric catalysis, namely bi-functional asymmetric catalysis like enzyme. That is the most significunt point of our research.
(Thank you very much.)
You are welcome.